How immune cells really migrate
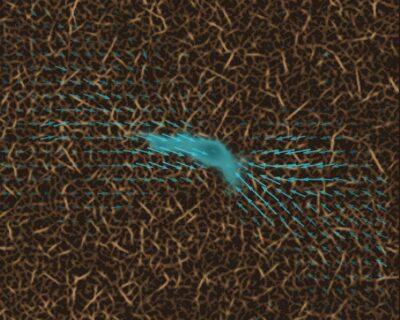
In order to reach their target, for instance a tumor, immune cells have to leave the bloodstream or lymphatic vessels and migrate through connective tissue. Until now, scientists presumed that immune cells migrated through tissue by constantly changing their shape and therefore squeezing through the smallest pores and openings.
FAU researchers use new method to measure cellular forces
Using a new measuring method, researchers at Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) have now been able to determine that immune cells also exert traction on surrounding tissue in order to pull themselves through particularly tight pores. Their results were published in the journal Nature Physics.
In order to migrate from A to B, immune cells do more than just adjust their shape. Occasionally, they attach to their surroundings and exert forces on these surroundings in order to pull themselves forward. “These contractile phases help immune cells to move through particularly tight pores,” explains Prof. Dr. Ben Fabry, holder of the FAU Chair of Biophysics and co-author of the study entitled “Dynamic traction force measurements of migrating immune cells in 3D biopolymer matrices”. “Immune cells are much quicker and considerably smaller than most other cells in connective tissue. For this reason, we have so far failed to measure such traction forces in immune cells. Our discovery was made possible only thanks to new, considerably quicker and more sensitive methods that we have developed and continually improved over recent years in Erlangen.”
Research at the interface to mechanobiology
The new measuring method is 3D traction force microscopy, a three dimensional measurement of traction and its impact on tissue. This method even allows scientists to measure the tiny forces of growing nerve cells as well as the forces of larger cell structures such as tumors.
The interdisciplinary nature of the team that includes researchers from immunology, physics, mechanics and neurosciences shows that the findings gained from 3D traction force microscopy are not only relevant for an isolated discipline, but rather are of groundbreaking significance for all sciences with any connection to mechanobiology.
Prof. Fabry emphasizes: “Our discovery that immune cells can create high contraction forces for a brief period of time is only one example of how this new method will lead to fundamental discoveries. We were also able to show in our study, for example, that growing nerve cells, particularly those referred to as growth cones, can also exert contraction forces on their surroundings. That may prove to be of fundamental importance for the formation of nerve pathways, particularly in developing brains.”
Further studies required
The research results do not yet allow any predictions of future applications. Fabry and his colleagues believe, however, that the knowledge about contraction forces in immune, nerve or cancer cells may contribute to developing medicines aimed at furthering specific healing processes or suppressing the progression of illnesses.
In the meantime, the scientists at FAU have started work on another study funded by the German Research Foundation (DFG) aimed at investigating the precise molecular mechanisms of the migration of immune cells due to traction forces.
DOI: 10.1038/s41567-024-02632-8
Video: Two migrating immune cells in a collagen matrix
Further information:
Prof. Dr. Ben Fabry
Chair of Biophysics
Phone: + 49 9131 85 25610
ben.fabry@fau.de